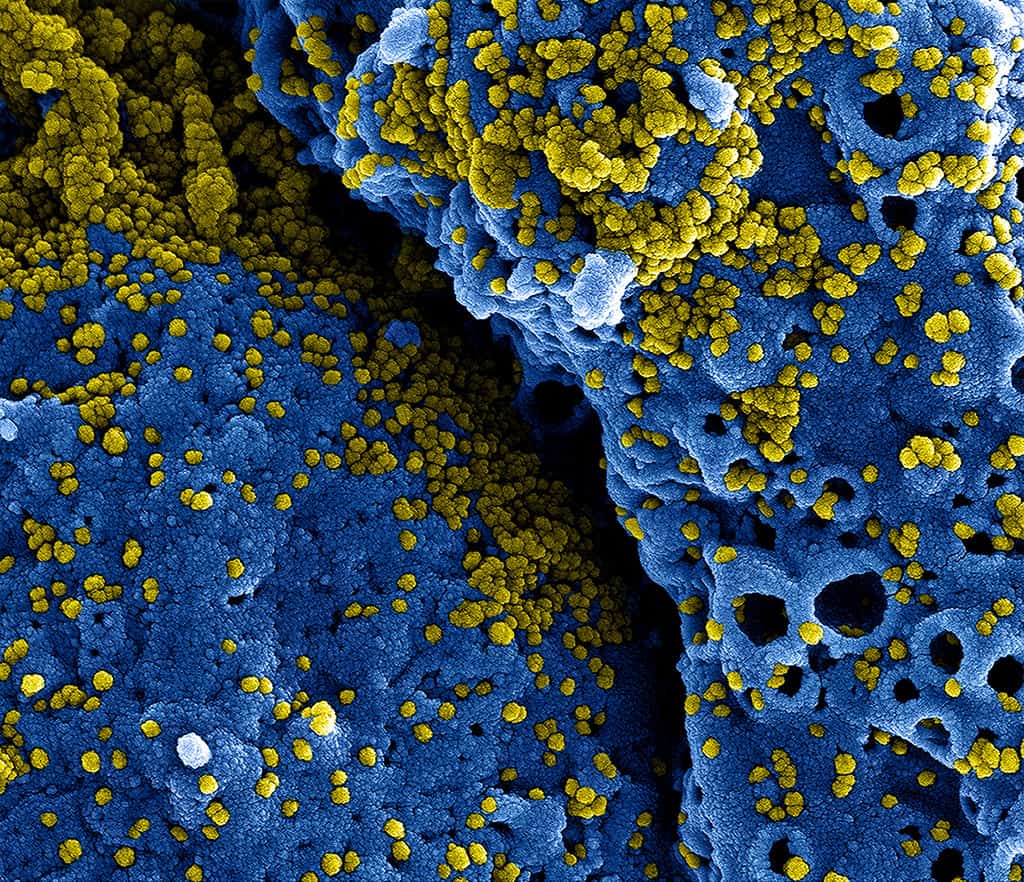
Stage 1 clinical testing of a COVID-19 vaccine has officially commenced in the Hubei province capital of Wuhan where the disease first broke out.
According to a report from Science and Technology Daily a total of 108 volunteers are participating in stage 1 testing, all of whom are permanent residents of the Wuhan area.
Participants received injections of the vaccine during the period from 18 – 20 March, before submitting to a 14 day quarantine and observation period.
The Academy of Military Medical Sciences and Hong Kong-listed CanSino Bio-B are responsible for holding the trials, according to information from the Chinese Clinical Trial Registry.
The stage 1 test participants are divided into three groups – a low dose group, a medium dose group and a high dose group. Each group is comprised of 36 volunteers.
According to Science and Technology Daily the vaccine was developed via genetic engineering, using replication-defective human type 5 adenovirus as a vector and expressing the new coronavirus S antigen.
Related stories
COVID-19 Vaccine Testing Commences in Wuhan
19 Chinese Drug Companies Working on Novel Coronavirus Vaccine
Coronavirus Vaccines Enter Animal Testing Phase: China’s Science Ministry